Learning Outcomes
By the end of this lesson, students will be able to:
i. Differentiate between shells and subshells, recognizing the hierarchical structure of atomic orbitals.
ii. Identify and describe the characteristics of s, p, d, and f subshells, the building blocks of electron configurations.
iii. Apply the Aufbau principle and the concept of subshells to write the electron configurations of the first 18 elements in the periodic table.
iv. Recognize the limitations of orbital diagrams and subshell notations, acknowledging the probabilistic nature of electron distribution.
v. Appreciate the significance of understanding subshells in comprehending the chemical properties and bonding behavior of elements.
Introduction
In the realm of atomic structure, electrons, negatively charged particles, occupy specific regions around the nucleus known as orbitals. These orbitals are grouped into energy levels, or shells, each characterized by a unique energy value. Within each shell, there exist subshells, further divisions that accommodate electrons based on their energy and angular momentum.
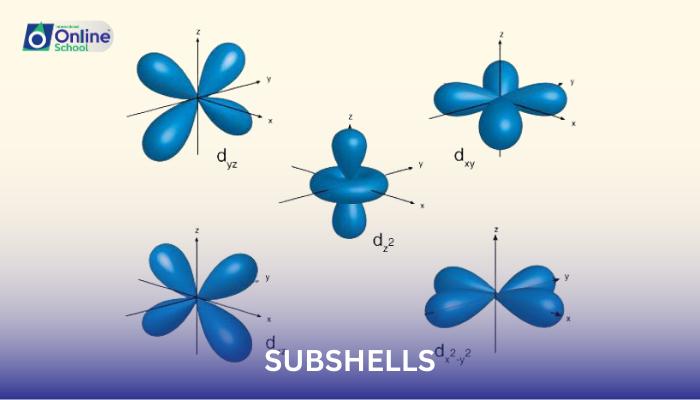
i. Shells and Subshells: A Hierarchical Structure
Shells represent the major energy levels within an atom, denoted by the principal quantum number (n). Subshells, on the other hand, are subdivisions within each shell, characterized by the azimuthal quantum number (l).
The four main types of subshells are s, p, d, and f, corresponding to l values of 0, 1, 2, and 3, respectively. Each subshell can accommodate a specific number of electrons: s subshells hold 2 electrons, p subshells hold 6 electrons, d subshells hold 10 electrons, and f subshells hold 14 electrons.
ii. Aufbau Principle and Electron Configurations
The Aufbau principle, a cornerstone of atomic structure, dictates the order in which electrons fill orbitals. Electrons fill orbitals of lower energy before moving to higher energy levels. Within each energy level, electrons fill subshells in a specific sequence: s first, then p, followed by d, and finally f.
Using the Aufbau principle and the concept of subshells, we can write the electron configurations of elements. For instance, the electron configuration of hydrogen (H) is 1s1, indicating one electron in the 1s subshell. Helium (He) has the electron configuration 1s2, with two electrons in the 1s subshell.
iii. Limitations of Orbital Diagrams and Subshell Notations
While orbital diagrams provide a visual representation of electron distribution, they have limitations. They do not accurately depict the probabilistic nature of electron movement or their precise locations within orbitals.
Subshell notations, though useful for representing electron configurations, do not provide information about the specific orbitals within a subshell that electrons occupy.
iv. Significance of Understanding Subshells
Understanding subshells is crucial for comprehending various aspects of an element's behavior:
Chemical Properties: The number of electrons in the outermost subshell, known as valence electrons, plays a significant role in determining an element's chemical properties.
Periodic Table Organization: Elements are arranged in the periodic table based on their electron configurations, with subshells contributing to the overall electron distribution.
Bonding Behavior: The type of subshells involved in bonding determines the nature of chemical bonds.
Subshells, the intricate subdivisions within atomic shells, provide a deeper understanding of electron distribution and its implications for an element's properties. By delving into the concept of subshells, we gain insights into the chemical behavior and bonding patterns of elements, laying the foundation for further exploration in the fascinating world of chemistry.